
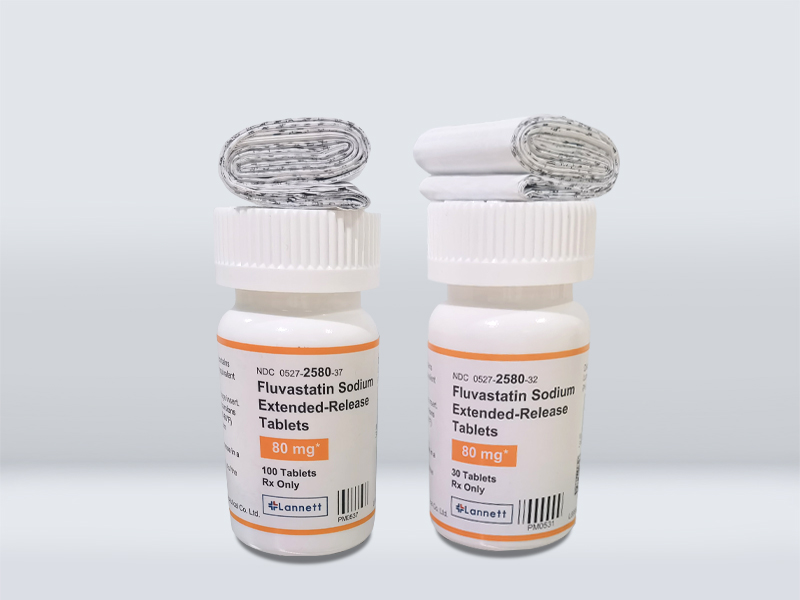
Product Name 産(chǎn)品名(míng)稱 | Fluvastatin Sodium Extended-Release Tablets 氟伐他(tā)汀鈉緩釋片 |
Strength 規格 | 80mg
|
Product License | ANDA # 209397
|
Indications 适應症 | As an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia and heterozygous familial hypercholesterolemia (HeFH) who require 80 mg 用(yòng)于飲食未能(néng)完全控制的原發性高膽固醇血症和混合型血脂異常的患者。 |
Approved Date 批準日期 | 4/26/2021
|
Market Authorization Holder 持有(yǒu)人 | Beijing Sciecure Pharmaceutical Co., Ltd. 北京世橋生物(wù)制藥有(yǒu)限公(gōng)司 |
Manufacture 生産(chǎn)商(shāng) | Beijing Sciecure Pharmaceutical Co., Ltd. 北京世橋生物(wù)制藥有(yǒu)限公(gōng)司 |