
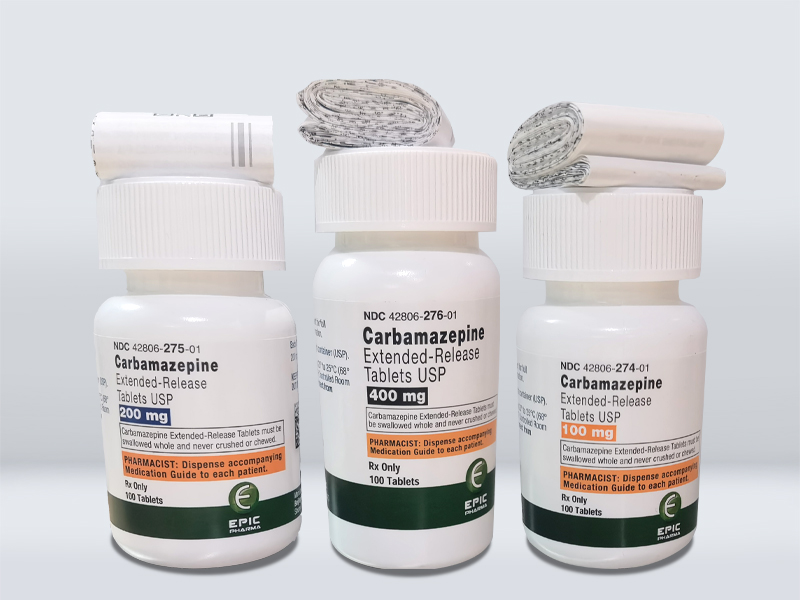
Product Name 産(chǎn)品名(míng)稱 | Carbamazepine Extended-Release Tablets 卡馬西平緩釋片 |
Strength 規格 | 100mg, 200mg, 400mg |
Product License | ANDA #216235 |
Indications 适應症 | Epilepsy: ①Partial seizures with complex symptomatology (psychomotor, temporal lobe). ②Generalized tonic-clonic seizures (grand mal). ③Mixed seizure, or other partial or generalized seizures. Trigeminal Neuralgia indicated in the treatment of the pain associated with true trigeminal neuralgia. Beneficial in glossopharyngeal neuralgia. 癫痫: ①症狀複雜的部分(fēn)性癫痫發作(zuò)(精(jīng)神運動、颞葉)②全身強直陣攣性癫痫發作(zuò)(大發作(zuò))③混合性癫痫發作(zuò),或其他(tā)部分(fēn)或全身性癫痫發作(zuò) 三叉神經痛: 用(yòng)于治療與真正相關的疼痛,在舌咽神經痛方面也有(yǒu)益。 |
Approved Date 批準日期 | 3/02/2023
|
Market Authorization Holder 持有(yǒu)人 | Sciecure Pharma Inc.
|
Manufacture 生産(chǎn)商(shāng) | Beijing Sciecure Pharmaceutical Co., Ltd. 北京世橋生物(wù)制藥有(yǒu)限公(gōng)司 |