
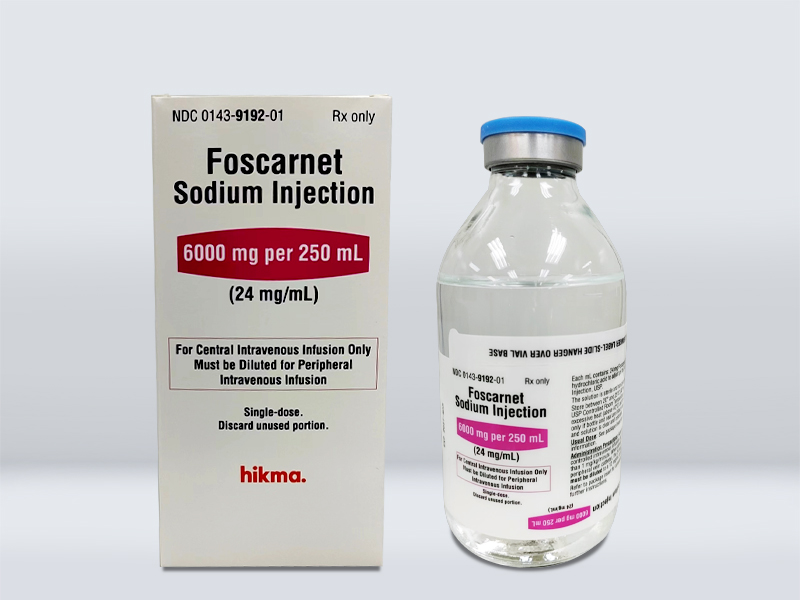
Product Name 産(chǎn)品名(míng)稱 | Foscarnet Sodium Injection 膦甲酸鈉注射液 |
Strength 規格 | 6000 mg/250 mL (24 mg/mL) |
Product License | ANDA #213987 |
Indications 适應症 | CMV Retinitis Mucocutaneous Acyclovir ResistantHSVInfectionsCMV視網膜炎 粘膜皮膚阿昔洛韋耐藥性HSV感染 |
Approved Date 批準日期 | 11/29/2023 |
Market Authorization Holder 持有(yǒu)人 | Beijing Sciecure Pharmaceutical Co., Ltd. 北京世橋生物(wù)制藥有(yǒu)限公(gōng)司 |
Manufacture 生産(chǎn)商(shāng) | Beijing Sciecure Pharmaceutical Co., Ltd. 北京世橋生物(wù)制藥有(yǒu)限公(gōng)司 |